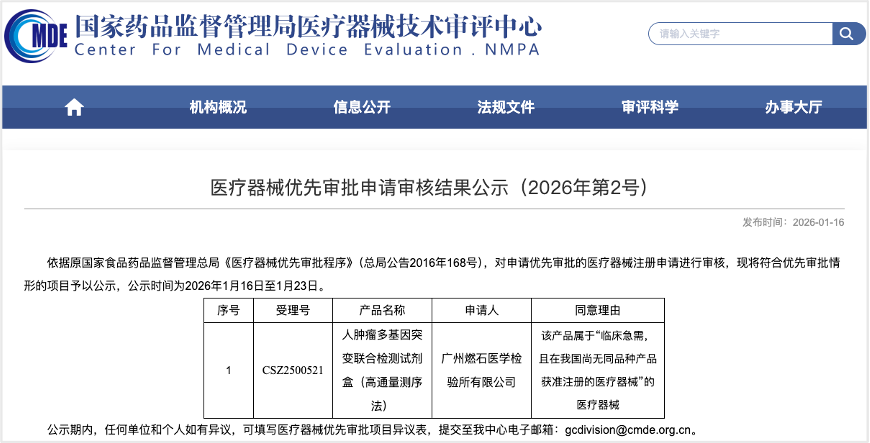
We are proud to announce that Burning Rock Dx’s self-developed "OncoScreen® BCMatch Tissue Kit" has officially entered the Priority Review Channel of the Center for Medical Device Evaluation (CMDE) under the NMPA.
As the first breast cancer NGS companion diagnostic (CDx) kit in China, this milestone significantly accelerates the domestic approval process and further reinforces our "One Kit, Global Partnership" strategic vision.
A Proven Track Record of Excellence: Byleveraging our leading NGS technology and extensive project experience, Burning Rock Dx continues to set industry benchmarks in the CDx field.
□ Partnership with AstraZeneca: The Capivasertib CDx module of this kit was approved by Japan’s MHLW in September 2025 and is now covered by Japan’s national health insurance—marking the first overseas NGS CDx approval for a Chinese company in the breast cancer field.
□ Partnership with Dizal Pharma: Our Sunvozertinib CDx kit was the first lung cancer NGS CDx approved by the NMPA under the new CDx guidelines, providing a breakthrough solution for NSCLC patients.
Actively Responding to Clinical Needs:Leading clinical guidelines, such as CSCO and NCCN, increasingly emphasize the critical importance of NGS technology in enabling personalized treatment and standardized clinical practice. By developing this NGS-based kit, Burning Rock Dx is directly responding to these clinical needs and policy orientations, ensuring high-quality diagnostic tools for the global oncology community.
With a network of over 140 pharmaceutical partners and dual CAP/CLIA-certified laboratories in both China and the US, we have successfully completed 310+ external quality assessments to ensure global consistency in diagnostic results.