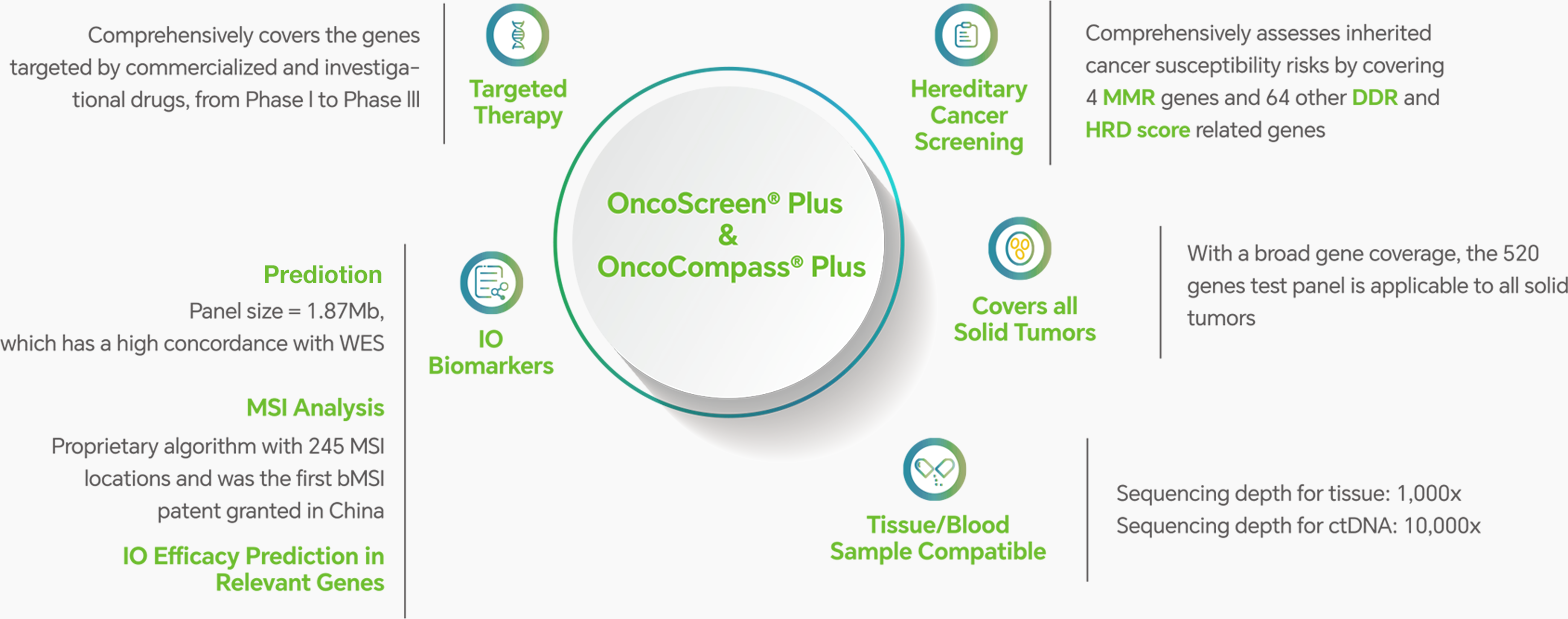

● Note: Different mutation types demonstrated a consistent >97% sensitvity and >95% specificity in clinical sample validation tests.


OncoCompass® Plus was utilized as the designated NGS assay in China for a multi-regional clinical trial (MRCT) in the HR+ advanced breast cancer, in which another NGS platform was used in other regions. Its inclusion reflects the assay’s analytical robustness and alignment with global clinical trial standards.