CanCatch® Leverages genomic and epigenomic profiling to offer diverse MRD detection Solutions.
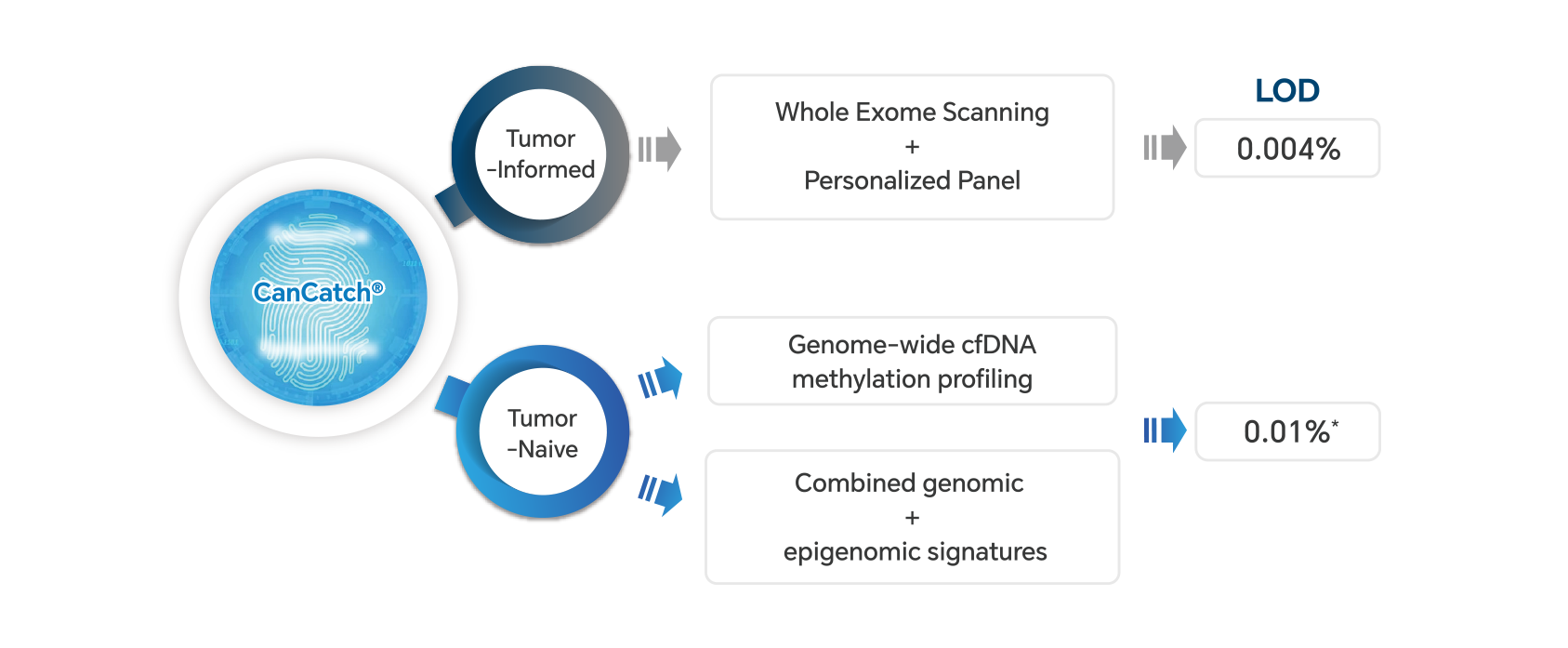
* : cfDNA down to 0.01%; Cell lines down to 0.004%

MEDAL Study1: Tumor Informed MRD
● CanCatch® personalized MRD assay outperforms fixed-panel mutation assays in NSCLC in the MEDAL Study.
● Post-relapse ctDNA status aids in guiding subsequent treat ment decisions.

Methylation-based MRD2,3
● The ELSA-seq technology integrates single-base methylation sequencing, multi-layer noise reduction, and machine learning to detect ctDNA at levels as low as 0.01%.
1. Chen et al., 2023, Cancer Cell 41, 1-14, October 9, 2023
2. Liang et al., 2021,Nature Biomedical Engineering 5, 586-599,1 June 15, 2021
3. Internal data.