Burning Rock offers integrated companion diagnostic development services for biopharma partners to streamline the co-development and commercialization of targeted drugs and respective CDx (including registration testing, clinical evaluation and finally obtain registration certificates). Our US-based lab and R&D team provide additional services to promote drug and CDx co-development worldwide.

-
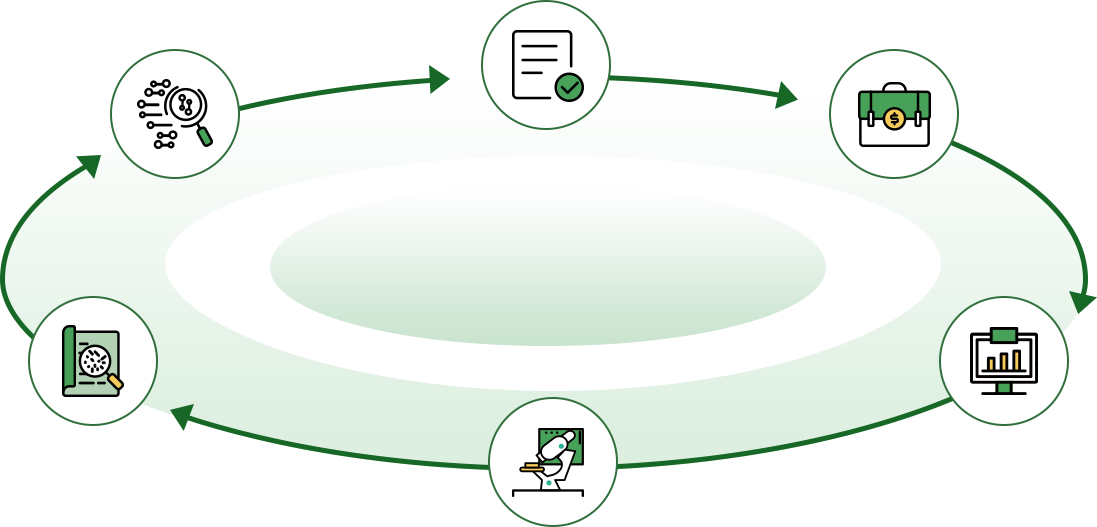
Product development
-
Registration testing
-
Clinical validation
-
Product registration
-

CDx overseas development
Product development and registration (FDA)
- Product development
- Raw material selection and preparation
- Production procedure evaluation
- Technical requirements proposal
- Product stability evaluation
- Product validation analyses
- Cut off value and coverage assessment
- Standardized production procedure
- Registration testing
- Document review
- Sampling and submission to testing agency
- Testing and data review
- Testing report
- Clinical validation
- Validates whether the product can meet its intended use
- Clinical accuracy: safety and effectiveness
- Intended use as a CDx product
- Product registration (NMPA)
- Document review
- Technical evaluation
- Inspection of the applicant’s quality management system
- Submission of supplementary materials
- Approval and certification
- Registration alteration (if applicable) or renewal
Precision companion
diagnostic strategies
- Product development and registration (FDA)
- In hospital marketing channels
- Online marketing promotion channels
- CDx developmnet
- CDx validation
- Patient Screening
